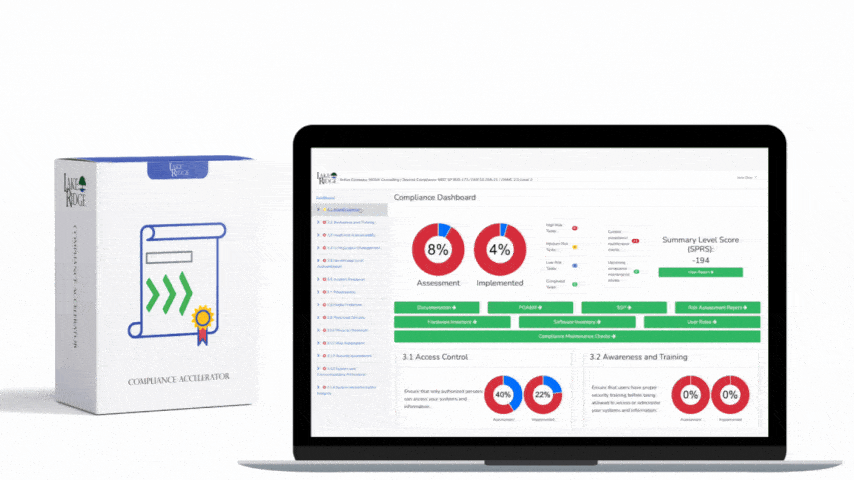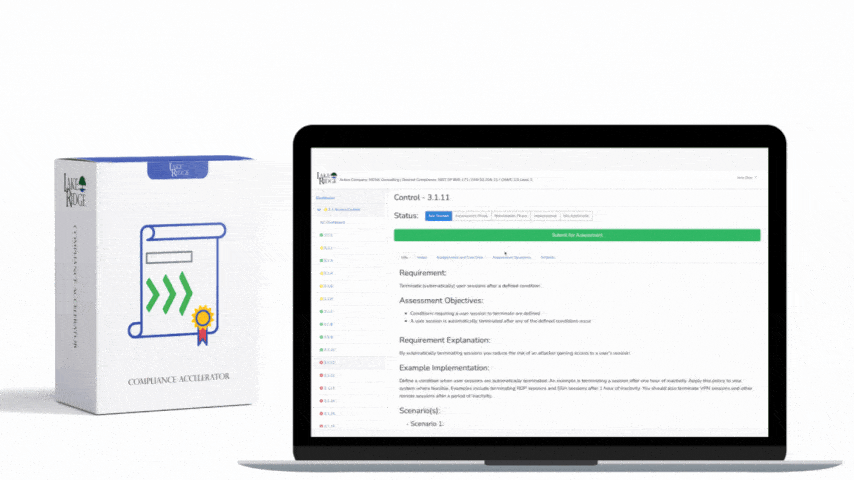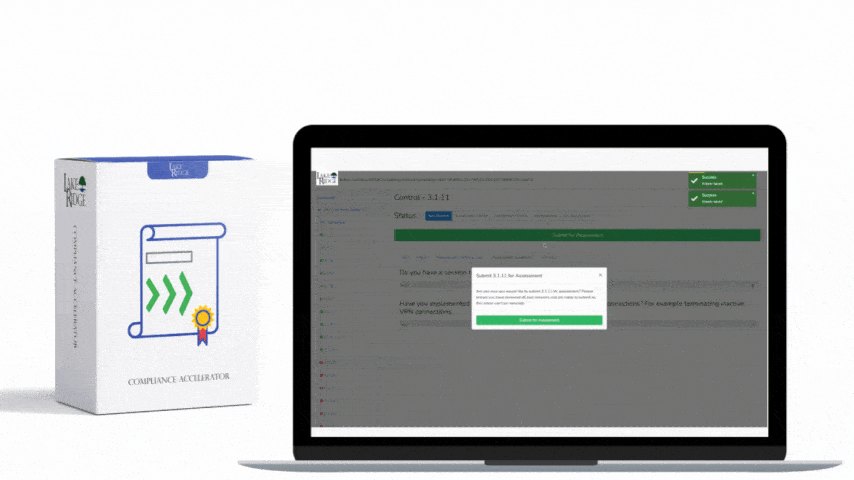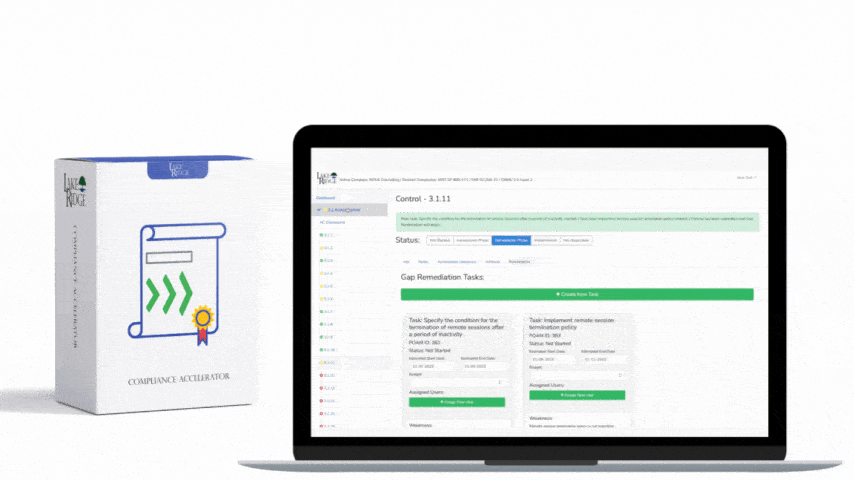
HIPAA 45 C.F.R. §164.316(b)(1) requires covered entities and business associates to have written policies and procedures and to maintain documentation of their implementation — a foundational requirement for any Compliance Framework program that wants to demonstrate consistent, auditable handling of protected health information (PHI).
Understanding 164.316(b)(1) in the context of a Compliance Framework
At its core, 164.316(b)(1) means your organization must (1) develop written policies and procedures that address HIPAA requirements, (2) implement them, and (3) retain evidence that they are current and followed. In a Compliance Framework this maps directly to Program Management controls (policy creation and approval), Implementation and Operations controls (enforcement, training, technical alignment), and Evidence and Audit controls (versioning, attestations, and records retention). The rule also implies a records-retention requirement: retain documentation for six years from creation or last effective date.
Practical implementation checklist — step-by-step
1) Assign ownership and define scope
Designate a HIPAA Privacy Officer and a HIPAA Security Officer (can be the same person for very small organizations) and identify policy owners for each domain (e.g., access control, incident response, data retention). For a small dental practice, the practice manager might be the Privacy Officer and the IT vendor the Security Officer; explicitly document those roles in the policy manual and include contact information and escalation paths.
2) Inventory PHI flows and map policies to processes
Create a simple data flow diagram that shows where PHI is created, stored, transmitted, and disposed. Use this to define policy coverage: minimum necessary, access control, encryption-at-rest/transit, remote access, BYOD, email/telehealth, and disposal. For a 10-provider clinic, map the EHR, practice management system, patient portal, lab interfaces, and cloud backups so each policy cites the systems and responsibilities.
3) Write clear, actionable policies and corresponding procedures
Policies should be high level and approve organizational positions; procedures must be step-by-step operational instructions. Example: Policy — "Access to EHR is role-limited and provisioned upon manager approval"; Procedure — "To provision EHR access: submit access request form, manager approves via e-signature, IT creates account, enables MFA, logs provisioning event." Include templates for requests, change forms, and BAA verification checklists.
4) Version control, approval, and distribution
Keep a single source of truth (Confluence, SharePoint, or a controlled document repository) with enforced versioning. Each revision should record author, approver, effective date, and reason for change. Implement an approval workflow: policy author → Security/Privacy Officer review → executive approval. Distribute with mandatory attestation for staff on initial release and after substantive changes; retain attestations as evidence.
5) Review frequency, change control, and retention
Establish a review cycle (at least annually or sooner when laws/technology change). Use change control to capture updates when new systems are introduced, a breach occurs, or OCR guidance changes. Retain policies and evidence of implementation (attestations, training records, audit logs) for six years from the last effective date to satisfy documentation requirements.
Technical details and small-business scenarios
Include specific technical requirements in procedures so implementers know what to configure: require AES-256 encryption for PHI at rest, TLS 1.2+ for PHI in transit, enforce MFA for remote access, enable detailed audit logging in the EHR and retain logs in a tamper-evident storage for at least 1 year operationally and ensure availability for forensic review (longer retention for evidence as required). For a small telehealth startup using S3, specify server-side encryption with a KMS-managed key, bucket policies that restrict access by VPC endpoint, and lifecycle rules that securely delete objects per retention policy. Document the exact configuration screenshots or CLI commands in the procedure to make audits straightforward (e.g., "Enable AWS S3 SSE-KMS with key arn:aws:kms:... and set versioning and MFA-delete").
Compliance tips, best practices, and the risk of non-implementation
Best practices: keep policies concise and role-focused, automate attestations via an LMS, use templates mapped to your Compliance Framework controls, and schedule tabletop exercises for your incident response procedure. Real-world small-business example: a 6-person counseling practice implemented a "Remote Work" procedure that required encrypted laptops, company-managed VPN, and daily backups; after a staff laptop theft the documented procedure and backup logs allowed rapid containment and full data recovery, and simplified reporting to OCR. Risks of not implementing written and maintained policies include inability to demonstrate compliance during an OCR audit, larger fines (OCR enforcement can include civil monetary penalties and corrective action plans), higher breach impact due to inconsistent practices, loss of business associate trust, and reputational damage.
Actionable next steps: create a policy register, assign owners within 30 days, draft key policies (Access Control, Incident Response, Data Retention, Business Associate Management) within 60 days, and complete attestations and technical configuration evidence within 90 days. Use simple tools that provide audit trails (GRC platforms, Confluence with restricted editing, or a Git repo for technical procedures) and keep an evidence binder that includes version history, training logs, BAAs, and log extracts.
In summary, meeting 164.316(b)(1) means more than writing documents — it requires assigning clear ownership, mapping policies to real processes and systems, enforcing procedures through technical and administrative controls, preserving evidence (including attestations and audit logs) for at least six years, and embedding review/change-control into your Compliance Framework. For small businesses, pragmatism matters: prioritize the highest-risk processes, document concrete steps (with screenshots or commands), and keep proof of implementation readily accessible for audits or incident response.