HIPAA 45 C.F.R. §164.316(a) requires covered entities and business associates to implement written policies and procedures and to maintain documentation demonstrating those policies were followed or why they were not; building audit-ready policies under a Compliance Framework is about producing reproducible, traceable documents plus the evidence trail that an auditor expects to see.
Understand the requirement and map it to your Compliance Framework
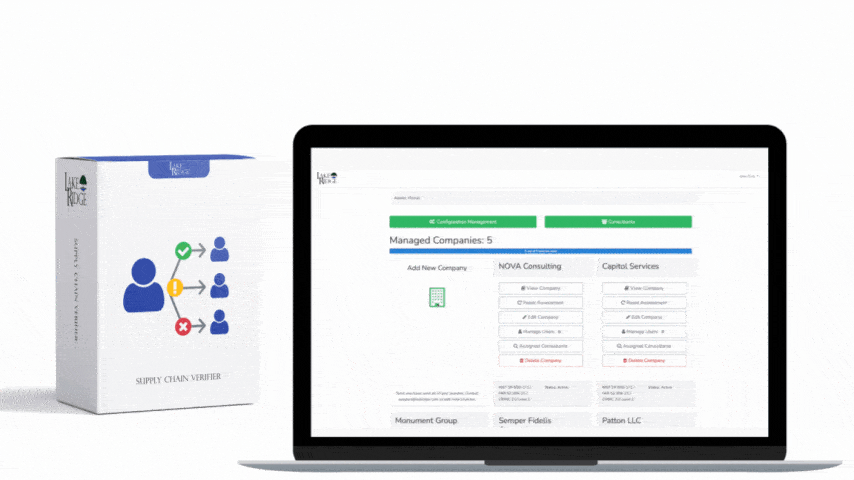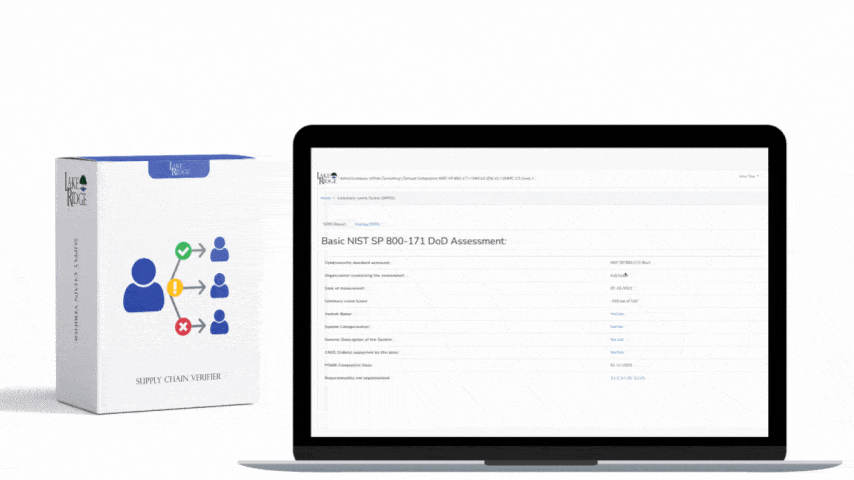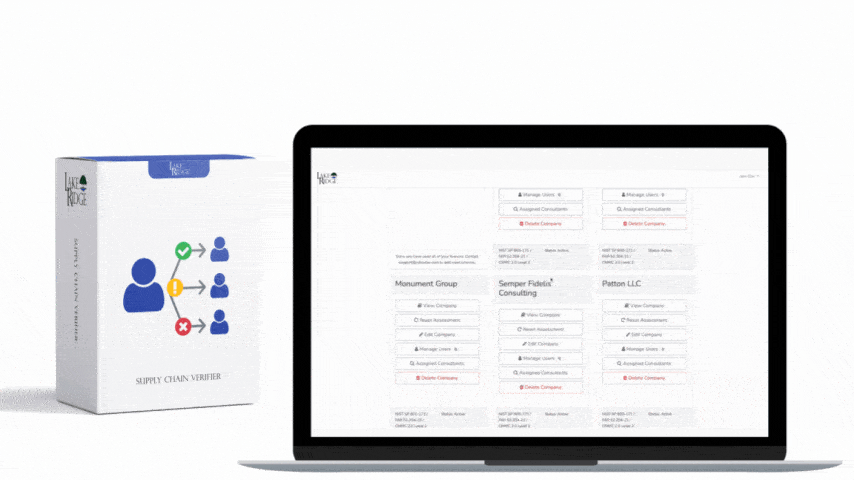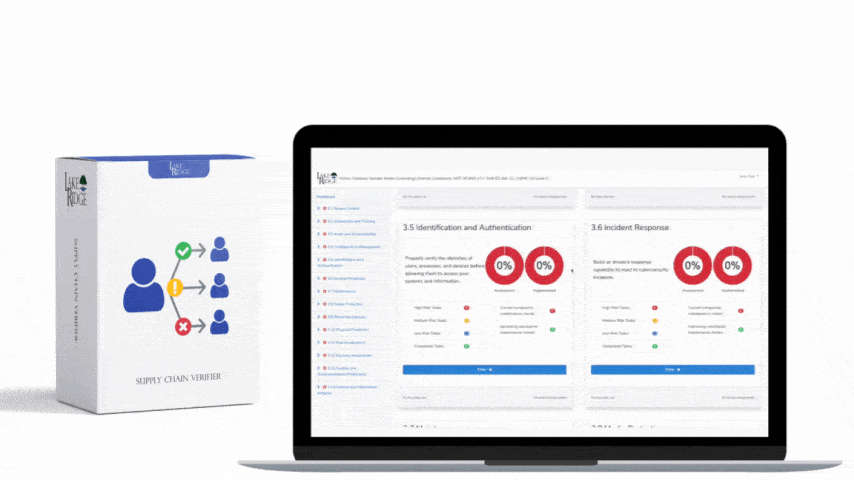
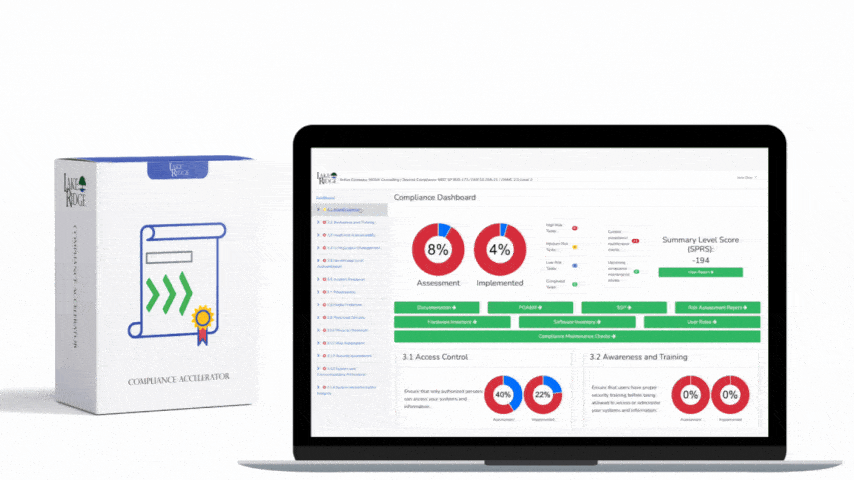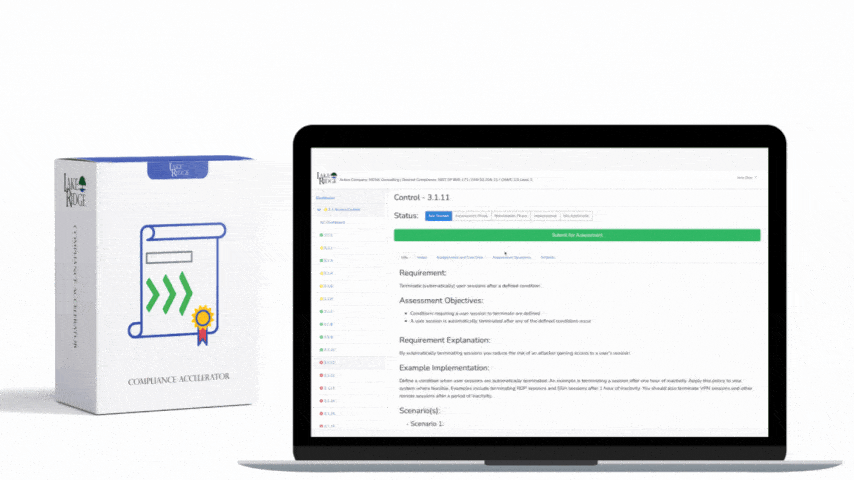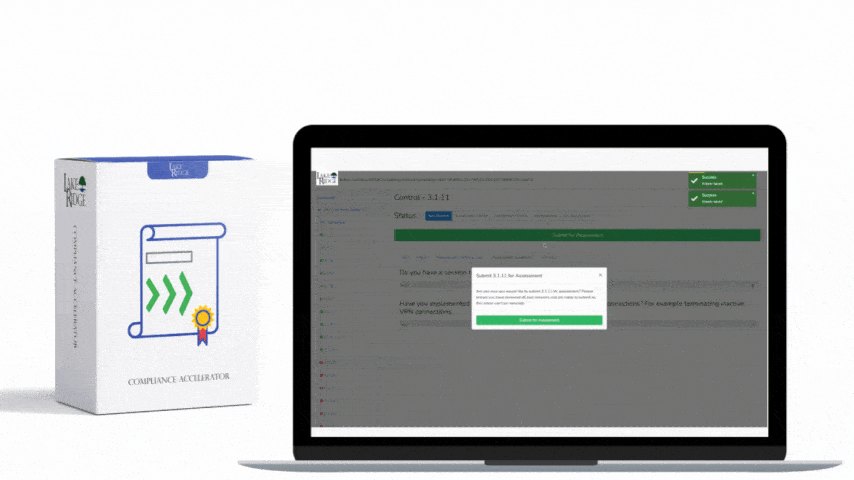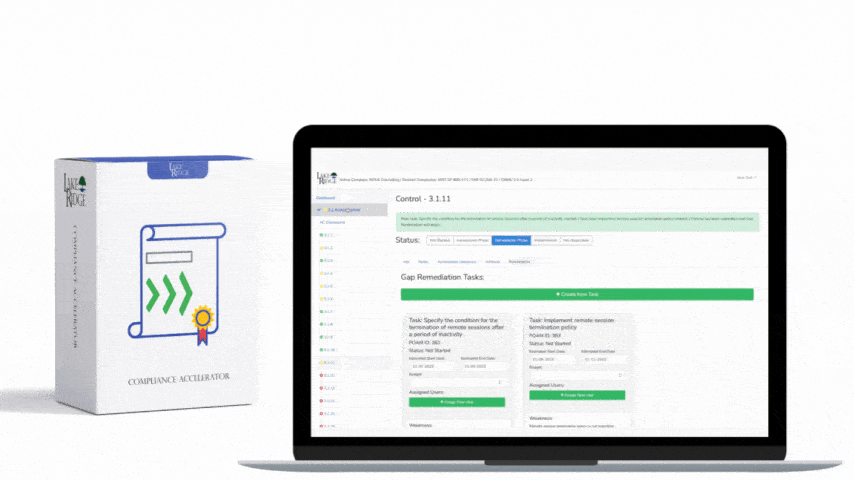
Start by mapping HIPAA 164.316(a) to the control taxonomy in your Compliance Framework: identify which framework control(s) require documented policies and procedures, assign owner(s), and link each policy to corresponding technical and administrative controls (for example: CF.Policy.P010 = HIPAA 164.316(a) — Policies & Procedures). Remember HIPAA’s retention expectation: keep documentation for at least six years from creation or last effective date. Document this retention in your framework’s metadata for each policy so retention and disposition are auditable.
Policy and procedure templates — what to include
Create standard templates inside the Compliance Framework repository so every policy looks and functions the same. Required template fields should include: Title, Control ID (framework mapping), Purpose, Scope (systems, locations, covered persons), Policy Statement, Responsibilities (role name and title), Step-by-step Procedures (SOPs), Exceptions and Approval Process, Monitoring and Reporting, Training Requirements, Metrics/Indicators, Retention, Glossary, Effective Date, Version, Change Log (who/when/why), and Approval Signatures. Use a consistent versioning scheme (e.g., vYYYY.MM.DD or semantic like v2.1.0) and require a mandatory approval signature field populated before a policy becomes “in effect.”
Template technical details
For technical procedures (SOPs), include exact configuration snippets, CLI commands, URLs, API endpoints, sample log queries, and expected outputs. Example: for encrypting PHI in transit, specify TLS 1.2+ required, accepted cipher suites, and the exact header/cipher config for your load balancer or reverse proxy. For data-at-rest, specify key management (e.g., KMS key ARN), encryption algorithm (AES-256-GCM), and where the encryption policy is enforced (application, DB, object store).
Documentation and the evidence you must collect
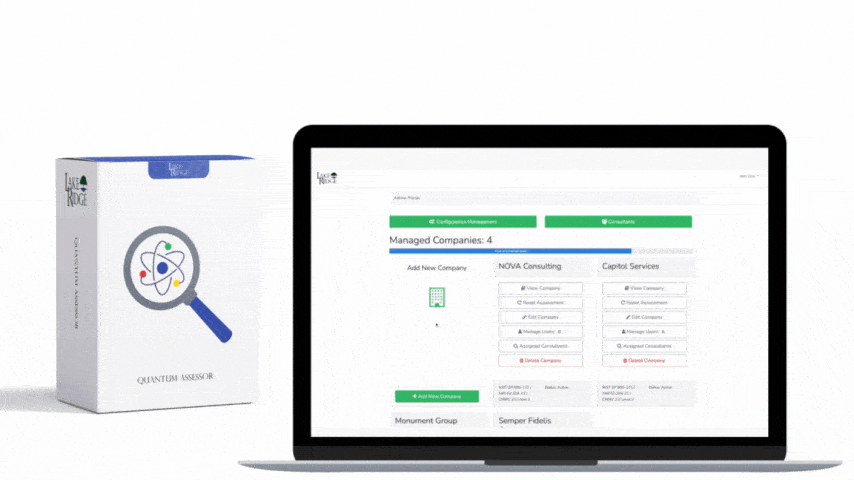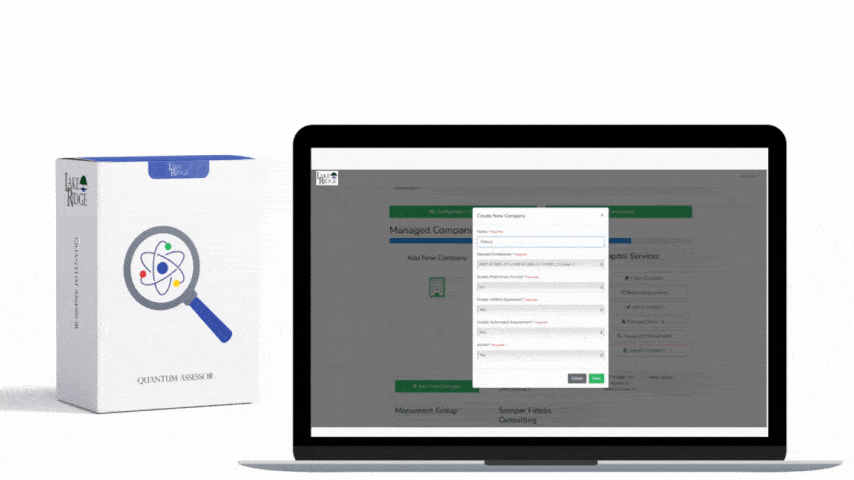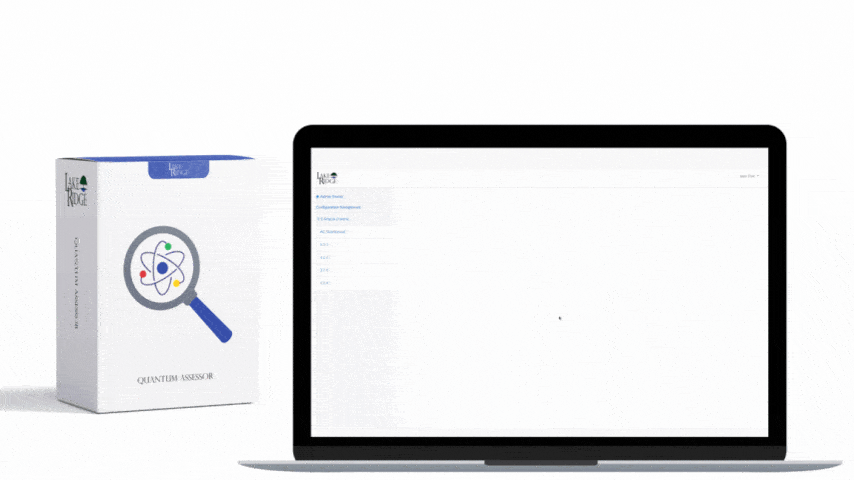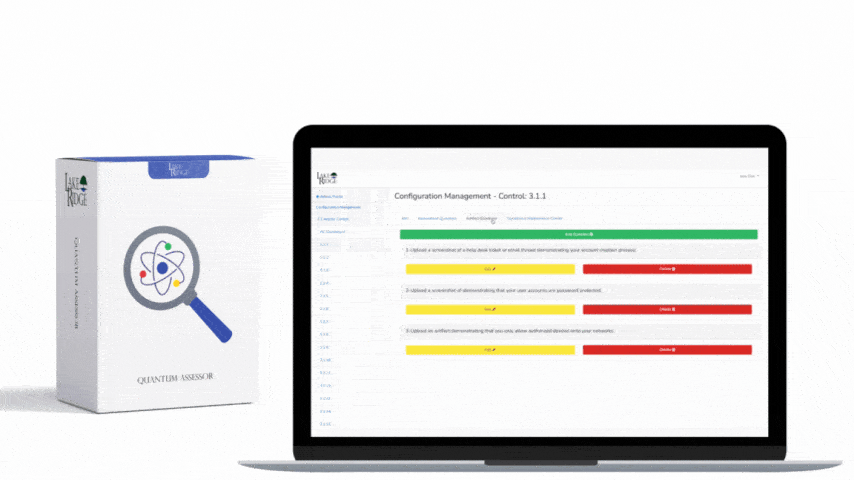
An audit-ready repository contains the policy document plus evidence artifacts mapped in the Compliance Framework: signed policy PDF, published URL, distribution list or who acknowledged, training completion records, review minutes, risk assessment that justified the control, change requests, and implementation evidence (screenshots, config backups, access control lists, firewall rules). Store originals in a secure document store with immutable versioning (for example: SharePoint with retention and version history, a Git repository with signed commits for code/SOPs, or cloud storage with object versioning and server-side KMS encryption). Maintain an audit trail showing who viewed/edited a policy and when — use timestamps and hashes (SHA-256) for key artifacts so auditors can verify integrity.
Change control — make it formal, testable, and traceable
Design a change control workflow inside your Compliance Framework that includes: a unique change request ID, purpose and scope, risk impact assessment (security, availability, confidentiality), test plan, rollback plan, implementation window, approver(s) including a privacy/security SME, and post-change validation steps. Record each step in a ticketing system (JIRA, ServiceNow, or equivalent) and link the ticket to the policy document’s change log. Require evidence attachments: test runbooks, validation logs, monitoring alerts, and a post-implementation review signed by the approver.
Emergency and out-of-cycle changes
Define an emergency change procedure: allow emergency implementation with a designated emergency approver (e.g., CISO/IT Lead) but require a documented retrospective (within 48–72 hours) that includes the reason for urgency, the temporary guardrails applied, and a timeline to produce a permanent policy update. The post-facto review must update the policy and the Compliance Framework mapping before the emergency change is accepted as permanent.
Real-world small business scenarios
Scenario 1: Small clinic (10–12 staff) adopting a new EHR vendor. Implementation steps: perform a gap analysis against existing policies, update the Access Control and Data Handling policies to include the vendor, get a signed BAA, document configuration specifics (vendor SSO SAML settings, minimum password complexity), store the policy and BAA in a secured SharePoint library with MFA and version history, and require staff to complete a short e-learning module with an acknowledgment that’s stored in the Compliance Framework.
Scenario 2: A telehealth rollout at a rural practice. Update Telehealth SOPs with explicit steps for secure video settings (end-to-end-like encryption where possible), patient consent language, temporary data storage windows, and a change control ticket that captures the integration testing results with the clinic’s firewall/NAT rules and the vendor’s media relay. Keep sample call logs (redacted), packet capture summaries for one test call, and a signed approval to demonstrate the controls were validated.
Compliance tips, best practices, and risk of not implementing
Best practices: assign a single policy owner for each policy, schedule annual reviews (or sooner for technology changes), automate retention and archival, require documented sign-off for any exceptions, and map each policy to measurable indicators (e.g., percent of staff with current training). Use automation: integrate ticketing with your document repository so a change request automatically links artifacts. Risks of not implementing: increased likelihood of PHI breaches, failure in an OCR investigation, monetary penalties, mandatory corrective action plans, lawsuits, and reputational damage. For small businesses a single breach can both incur steep costs and permanently damage patient trust.
Summary: Treat HIPAA 164.316(a) policies as living, auditable artifacts within your Compliance Framework — use standardized templates, collect and preserve implementation evidence (signed policies, configuration snapshots, training records), and operate a formal change control process that includes emergency handling and retrospective reviews. Doing this will make your program demonstrably audit-ready, reduce regulatory risk, and provide a clear operational path for both routine and out-of-cycle changes.